Discussion of the equation for salt pH calculation
Equation 11.13 derived in salts in general section is one of the most interesting and universal we have seen to this time. Almost all problems solved before are just its particular cases.
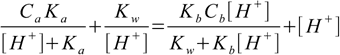 14.1
14.1In the case of mixture of strong acid and strong base both dissociations constants are large and we can assume Ka>>[H+], Kb[H+]>>Kw. That means that in both sums in denominators smaller values can be neglected. After canceling out what we are left with is
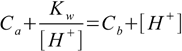 14.2
14.2which is a more universal form of equations 7.4 and 7.7 - this time it describes solution containing both strong acid and base, regardless of their concentrations. Even for diluted solutions results will be correct, as water dissociation is taken into account thanks to Kw presence. Putting Ca=0 or Cb=0 we are getting quadratic equations for pH of strong base or acid.
In case of solution of salt of strong acid and strong base Ca=Cb and these cancel out, leaving the simplest possible thing:
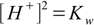 14.3
14.3We can also put Cb=0 to get
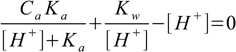 14.4
14.4which is nothing else but equation 6.8 describing pH of any acid solution.



