pH lectures - the Arrhenius theory of acids and bases
The simplest and most straightforward theory of acids and bases was proposed by a Swedish scientist, Svante Arrhenius, in 1884. According to his definition, acids are substances that produce H+ in solutions:
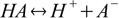
while bases dissociate giving off OH-:
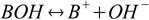
At the time Arrhenius published his theory, it was assumed H+ is a free ion roaming in the solution. Later research showed H+ is not alone, but is surrounded by water molecules, creating a hydronium ion (H3O+) or even larger ions (H5O2+, H7O3+ and so on). However, it doesn't change the general idea of Arrhenius acids and Arrhenius bases being substances dissociating into H+ and OH- and some counterion.
Arrhenius theory nicely explains why many substances - like HCl, H2SO4, HNO3, NaOH, KOH, Ca(OH)2 - can be classified as either acids or bases, it also explains neutralization reaction between acids and bases as a reaction between hydronium and hydroxide:
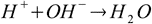
Arrhenius theory can be also used as a starting point for pH calculation of solutions containing acids and bases listed above (see introduction to acid/base equilibrium). It is still the starting point for the acid/base reaction discussion. However, soon it became obvious that Arrhenius theory is limited, as it fails to explain why substances like ammonia are basic, so in 1923 it was replaced by more advanced Bronsted-Lowry theory of acids and bases.



