pH definition, pOH definition
Every water solution contains H+ ions (technically there are no free protons in solutions, they are solvated creating larger ions like hydronium H3O+, H5O2+ and so on - as for calculations exact form in which these ions are present in the solution doesn't matter, we will always refer to H+). The H+ concentration is one of the most important parameters describing properties of water solutions.
The concentration of H+ can change in a very wide range, it can be 10 M as well as 10-15 M. Such numbers are inconvenient to use so to simplify things Danish biochemist Søren Sørensen developed in 1909 the pH scale and introduced pH definition - minus logarithm base 10 of [H+]:
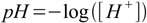 0.1
0.1It is much easier to use pH definition and to say "pH of the solution is 4.1" than to use concentrations - as in "H+ concentration is 0.000079M".
Idea of using letter p to denote concentrations and numbers that can vary by several orders of magnitude was widely accepted and is used not only in pH definition, but for example also for displaying dissociation constants values in tables.
Not only H+ ions are present in every water solution. Also OH- ions are always present, and their concentration can change in the same very wide range. Thus it is also convenient to use similar definition to describe [OH-]
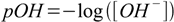 0.2
0.2Warning: In real solutions not concentrations, but ion activities should be used for calculations. Especially pH definition uses not minus logarithm of concentration, but minus logarithm of activity. In diluted solutions activity is for all practical purposes identical to concentration, but when the concentration goes higher activity starts first to be lower than the concentration, then - once the concentration rises - higher than the concentration. As a rule of thumb if the concentration of charged ions present in the solution is below 0.001 M you don't have to be concerned about activities and you can use classic pH definition. This will be addressed in more details in the ionic strength and activity coefficients section.



