Definitions of less popular dissociation constants types
We already know that to express acid (or base) strength we can use both acid and base dissociation constants, both stepwise and overall - they are interchangeable and it is always possible to convert between them. What is more important, they have all their uses - sometimes it is much more convenient to do the calculations using one form than the other.
However, these are not all types of constants used. Sometimes (although rarely, see for example Janos Inczedy book Analytical Applications of Complex Equilibria) protonation constants are used. What are these?
Consider dissociated acid H2A. There are A2- anions in the solution, and they can get protonated:
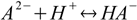
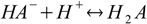
Stepwise protonation constants are defined as
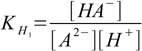 4.1
4.1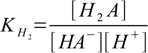 4.2
4.2and overall protonation constants as
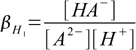 4.3
4.3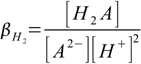 4.4
4.4They look strange and are rarely used in everyday practice, but they have one advantage that can be sometimes important: calculations performed with their use look exactly the same as the calculations of complex equilibria. Reason is simple - protonation is in a way similar to adding ligand to the complex.
Converting from protonation constants to dissociation constants is not very difficult even with pen and paper, but the easiest way is to enter them in BATE pH calculator database dialog window.

