Buffer lectures - buffer capacity examples
As we have seen calculating changes of pH of the buffer solution after adding strong acid or strong base, not all buffers are equal - depending on their composition some are more resistant to the added acid/base, some less resistant. Parameter that describes buffer resistance to pH changes quantitatively is called a buffer capacity. There are many ways of defining the buffer capacity, we will use the definition that most closely follows intuition - the buffer capacity should be larger for solution in which pH change is smaller after addition of acid/base, it should not depend on whether we are adding acid or base, and should be not a function of the amount of acid/base added, but rather should describe pH change in response to infinitesimally small amount of acid/base added. Thus it is best defined as a first derivative of n with regard to pH:
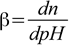 5.1
5.1It can be shown (see buffer capacity discussion in pH lectures, where the formula is derived) that buffer capacity of a solution containing several buffering agents, each described by Cbuff concentration and Ka dissociation constant, is
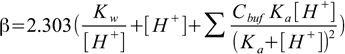 5.2
5.2Note, that even if the solution doesn't contain a buffer, for low and high pH it will have a high buffer capacity - while it may sound strange, it is perfectly correct. Low pH (or high pH) mean high concentration of a strong acid (base) - and such solution is quite resistant to pH changes (try to calculate by how much pH of the 1 L of 0.1 M HCl solution changes after adding 0.001 moles of a strong base - you don't need pKa for that, just stoichiometry of the neutralization and pH definition). For 0.1 M acetic buffer buffer capacity curve looks like that:
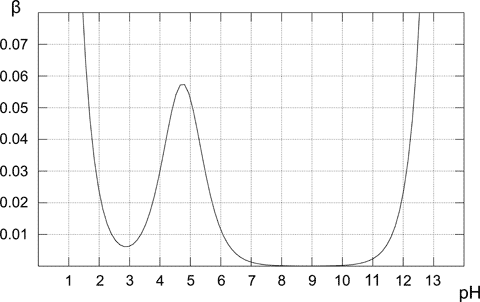
Note, that buffer capacity curves calculated and displayed by the Buffer Maker, usually don't cover whole pH range. That's because buffer capacity plot is calculated only for buffers that can be prepared from the chosen reagents, and possible pH range is usually limited to just several pH units.
Typically buffer capacities are in the 0.01-0.0001 range:
| solution | capacity |
|---|---|
| sea water | 2×10-4 |
| acetic acid/sodium acetate, pH=4.75, 0.001 M | 6.2×10-4 |
| bleach | 0.001 |
| extraction buffer for extracting lactate dehydrogenase from chicken meat | 0.003 |
| blood | 0.005 |
| acetic acid/sodium acetate, pH=4.75, 0.01 M | 0.0058 |
| solution with added ammonia buffer (as used in complexometric Mg titration) | 0.01 |
| Coca-Cola | 0.04 |
| acetic acid/sodium acetate, pH=4.75, 0.1 M | 0.058 |
| acetic acid/sodium acetate, pH=4.75, 1 M | 0.49 |
Remarks:
All these numbers are only approximate and were calculated using information about weak acids/bases present or required for the solution preparation:
- Capacity of the sea water was calculated assuming composition of an artificial standard sea water, without alkaline earth metals - their presence changes the capacity, but change was estimated as orders of magnitude lower than the value calculated.
- Buffer capacity for bleach was calculated as if it was just a 1 M solution of the sodium hypochlorite. Despite relatively high concentration buffer capacity is low, as pH is far from pKa.
- Concentration of HCO3- in blood is typically listed as 0.024 M. pH of blood is usually around 7.4 which allows calculation of H2CO3 to be around 0.0019 M, and these numbers were used for buffer capacity calculations.
- Buffering capacity for Coca-Cola was calculated from Pemberton recipe, which suggests around 0.07 M of citric acid, neutralized to pH of 2.7. Modern coke contains phosphoric acid, so its capacity is probably slightly different.
- Extraction buffer for lactate dehydrogenase from chicken: 10 mM Tris, 1 mM 2-Mercaptoethanol, 1 mM Phenylmethylsulfonyl fluoride (PMSF), 1 mM EDTA, pH adjusted with HCl to 7.4 (taken from CSUF Chemistry 422 lab manual by Mark Brandt, slightly modified after consulting with the author).
- For acetic buffer capacity is not a linear function of concentration - in the concentrated solution Ka value changes due to high ionic strength of the solution (so the solution at pH 4.75 no longer directly follows the pH=pKa rule), in the diluted solution concentration of free H+ starts to play an important role.



