preparing chemistry quizzes and tests - questions on pH calculation
This quiz was slightly different then the other two as it covered much broader scope of the curriculum, thus it required 4 calculation questions, of which one was an extra question for 6 (grades in Poland are on the 1 to 6 scale, with 5 being considered very good and 6 a super effort grade).
Teacher proposed the following questions:
- Calculation of a pH of the weak acid, but with too high dissociation degree for the most simplified equation (see pH of weak acid calculation).
- Calculation of pH of a salt of weak acid and strong base, in the range for the mentioned simplified equation (hydrolysis ratio below 5%).
- Reading pKa of the weak acid from the titration curve.
- Extra question: calculation of pH of mixture of diluted strong acid and strong base. Idea was to mix limiting reagent type problem with the necessity of taking water autodissociation into account.
We started with the first question. Wording was obvious:
What will be pH of the solution obtained by dissolving .... g of benzoic acid in 1L of water. pKa = 4.19. pH = ......
Only thing that required attention was correct selection of acid mass, so that dissociation degree will exceed 5%. We started pH calculator, created a new solution, selected benzoic acid from the database and opened equilibrium window, which lists concentrations of all forms of the acid present in the solution; dissociation degree will equal concentration of benzoate anion from dissociation. We have entered 0.1 as the acid concentration. Concentration of anion from the dissociation was immediately listed as 2.5% - too low. We changed concentration to 0.01, that gave dissociation degree of 7.7%, high enough for our needs. Clicking on the Ca button opened concentration calculator for benzoic acid - with 0.01 already copied as concentration from the edit field were it was entered. What we wanted to do was to find such mass of the benzoic acid, that dissolved in the 1 liter of solution will give concentration not higher than 0.01 M. We locked 0.01 as concentration and entered solution volume - 1 L. Concentration calculator showed that we need 1.2212 g of solid.
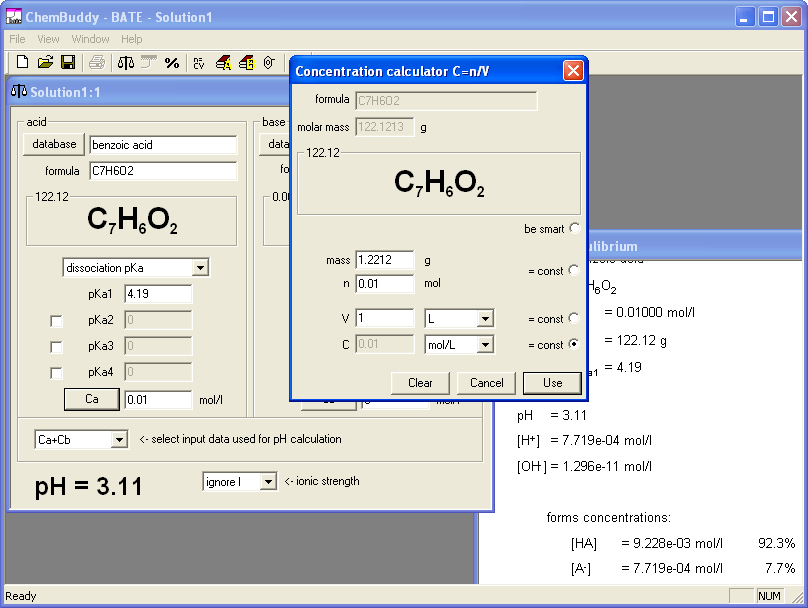
Obviously lower concentrations will have higher dissociation degrees. At this point we could either select six different concentrations lower than 0.01M or six different masses lower than 1.2212 g - in both cases time required for the question preparation will be the same. Idea that using "round" values for the concentrations will build confidence in students that they are doing OK prevailed, and we entered 0.010, 0.009, 0.008, 0.007, 0.006 and 0.005 as concentrations, copying in each case mass calculated by the BATE (respectively 1.2212, 1.0991, 0.9770, 0.8548, 0.7327 and 0.6107) into question document. Whole operation took much less time than writing about it. Also preparation of the answer key was very fast - BATE automatically calculates pH when concentration is entered, so all pH values were calculated and noted down in just a few seconds.
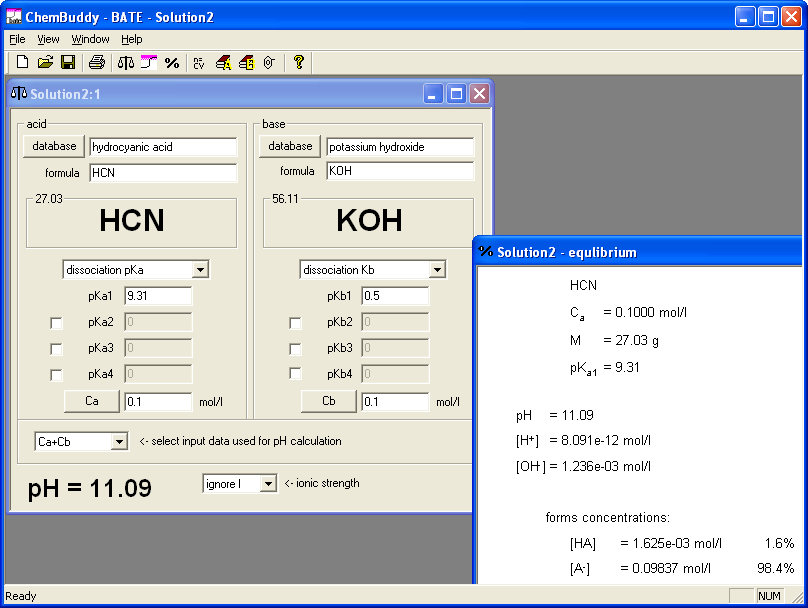
Work on the second question was very similar and based on the same approach. We created a solution containing equimolar concentrations of potassium hydroxide and hydrocyanic acid - that's the BATE way pf preparation of salt of weak acid and strong base. Again equilibrium view gave us information about hydrolysis degree. This time first entered value of the concentration was right - for 0.01 M solution dissociation degree is 4.5%; any higher concentration will do. Selected wording of the question was:
.... M solution of hydrocyanic acid (pKa = 9.31) was completely neutralized with solid KOH. What is pH of the solution? pH = .......
We decided to use 0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 as molar concentrations. Once again, to prepare answer key it was enough to enter concentrations of reagents - and it took just several seconds.
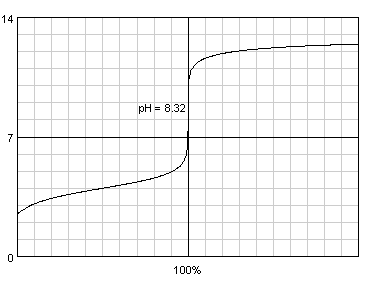
Third question can be prepared using BATE titration view, which - once both acid and base are selected and their concentrations are entered - shows both acid/base and base/acid titration curves. Titration curve displayed by the BATE can be copied to other documents by means of using clipboard (with PrtScr and any graphic program), but we had beta version of BATE 1.0.2, which saves titration curves directly to disk in PNG format. Note: this version of the program will be released once beta tests are over, which should happen later this year, but if you are interested don't hesitate to ask, we will gladly share; program is quite stable. We decided to use fake acids with pKa of 2.5, 3, 3.5, 4, 4.5 and 5. Once again, preparing all six titration curves and incorporating them into the questions document took about a minute, most time was spent outside of BATE. Note, that titration curves exported for quiz looked much better, the one presented here was exported in web quality, much lower than print quality.
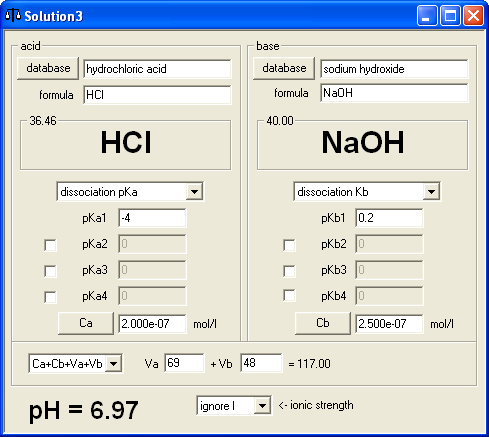
Finally, we came to the extra question. In this case it is not possible to tell how does BATE calculations result compare to the result of calculations that don't take water autodissociation into account. To be on the safe side we had to be sure that concentrations of acid and base used are in the range of 10-7 M. We decided to start with 69 mL of 2×10-7M hydrochloric acid. Concentration calculator showed that we have 1.380×10-8 moles of acid, so we decided to add between 1×10-8 and 2×10-8 moles of base - in six versions 1×10-8, 1.2×10-8, 1.4×10-8, 1.6×10-8, 1.8×10-8 and 2×10-8, using 3×10-7 M sodium hydroxide. Base solution volumes were calculated using concentration calculator - and once we started calculating volumes it was immediately apparent that 3×10-8 concentration wasn't a good idea, as first volume was 33.3(3) mL and such numbers are inconvenient to work with. Thus we changed concentration to 2.5×10-8 M and all volumes became integer - 40, 48, 56, 64, 72 and 80 mL. Question was worded as:
To 69 mL of 2×10-7 M hydrochloric acid .... mL of 2.5×10-7 M sodium hydroxide was added. What was the final pH of the solution. pH=........
Preparing answer key was almost immediate - one of the BATE modes of operations is calculation of pH of solution prepared by mixing solutions of acid and base of given concentrations, so it was just a matter of entering 6 volumes and copying displayed results (6.92, 6.97, 7.00, 7.04, 7.06 and 7.09 in case you want to know).
Day after we have prepared the quiz, teacher called us. She told us, that she wasn't confident our program works OK so she have checked all calculations manually, and the results for the extra question were wrong. We rechecked and compared BATE result with her results and to her embarrassment it turned out that she forgot to account for the dilution - to find concentration of acid/base she was calculating number of moles of substance left after neutralization and then dividing it by the volume of the acid (69 mL), while the final volume was higher.



