Calculate pH of 0.001M solution of benzoic acid, pKa=4.19.
We will start with the simplified formula 8.13.
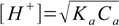 8.13
8.13From formula we get [H+]=2.54×10-4 M and pH=3.60. Is it correct? To be sure we have to check if the 5% rule is obeyed, that is if the dissociation fraction is less than 5%.
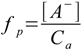 1.3
1.3Dissociated acid concentration is the same as [H+], thus dissociation fraction is 2.54×10-4/0.001 - above 25%. We can't use simplified formula 8.13! We have to use more precise equation 8.10:
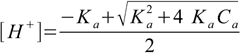 8.10
8.10This time we get pH=3.65, exactly the same result as from our pH calculator.



