Calculate pH of 1M solution of nitrous acid, pKa=3.37.
Let's try first with the simplified formula 8.13.
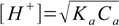 8.13
8.13If we put concentration and Ka values into formula we get [H+]=0.021 M and pH=1.69. Is it correct? To be sure we have to check if the 5% rule is obeyed, that is if the dissociation fraction is less than 5%.
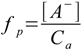 1.3
1.3Dissociated acid concentration is the same as [H+] - if we assume we can neglect water dissociation. It is obvious that we can, as 0.021 M is five orders of magnitude larger than the concentration of H+ from the water. So dissociation fraction is 0.021/1 - a little bit over 2%, much less than 5%, so the 5% rule is obeyed.
As additional confirmation our pH calculator shows exactly the same result.



