Volume-volume percentage definition
Volume/volume percentage (% v/v) is defined as
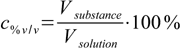 4.1
4.1For example wine contains about 12% v/v ethanol, which means there are 12 mL of ethanol in every 100 mL of wine.
The problem is volume is additive for ideal gases only. In all other cases final volume is not a sum of volumes used when preparing mixture, thus volume-volume percentages don't sum to 100%.
If you take 50 mL of ethanol and fill it up to 100 mL with water what you get is 50% v/v solution of ethanol in water. What is water v/v concentration in this solution? Logical answer seems to be 50%, but due to volume contraction to get 100 mL of solution you have to add 53.7 mL of water - so water concentration is 53.7% v/v and sum of both concentrations is 103.7% v/v. Not good.
Due to the volume contraction to convert between % v/v and other concentration types you will need densities of the solution and of both pure solvent and solute.
Volume-volume percentage is expressed in [%] units.



