Percentage to molality concentration conversion formula derivation
Molality conversions are more demanding, as we need to separate solvent and solute. Let's start with definitions of both molality and mass percentage:
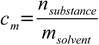 11.1
11.1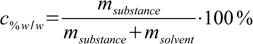 11.2
11.2At first sight there are no common points in both equations. For sure we will need additional information about molar mass of the substance.
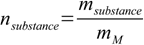 11.3
11.3Combining 11.1 with 11.3 we get
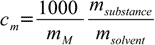 11.4
11.41000 factor is necessary as molality is expressed in [mole/kg] while all masses are in grams.
If we could rearrange our definition of weight percentage in such a way that that we will have msubstance/msolvent quotient on the left side of the equation, our conversion formula will be ready. Let's try. First, we multiply both sides of 11.2 by the msubstance+msolvent sum:
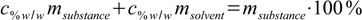 11.5
11.5Now let's group all terms containing both masses on both sides of equation:
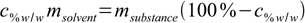 11.6
11.6Almost done:
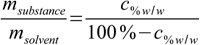 11.7
11.7And finally replacing msubstance/msolvent quotient in 11.4 by 11.7:
 11.8
11.8If you solve this equation for c%w/w you will get formula for opposite conversion - from molality to weight percentage.



