preparing chemistry quizzes and tests - stoichiometry questions
Our stoichiometric calculator is capable of balancing reactions and calculating stoichiometric amounts of reagents, regardless of whether their amount is given as mass, as number of moles, as ideal gas of given pVT parameters, or as solution of known concentration and volume. That makes it particularly convenient tool for preparing quizzes regardless of the students level of education.
Quiz that we prepared was administered at the end of (broadly speaking) d-block metals chemistry section, combined with redox reactions, thus questions had to cover both subjects. Note, that complete quiz was longer, we are only discussing questions that require calculations.
First question was selected by the teacher to be about iron (II) oxidation to iron (III) with potassium dichromate, with limiting reagent calculation:
Calculate amount of iron (III) sulfate produced, when ... mL of 1M potassium dichromate is mixed in excess sulfuric acid with 1.2 g of FeSO4. Answer: ......... g.
This particular reaction was not covered during lessons, so while the teacher expected students to be able to balance the equation, she wanted to give a hint in the form of a skeletal equation (alternatively, information about Cr3+ being one of the products should be enough).
We started with entering reaction as unformatted string - FeSO4 + K2Cr2O7 + H2SO4 = Fe2(SO4)3 + Cr2(SO4)3 + K2SO4 + H2O - using EBAS New from string command. That served two purposes. First of all, reaction was automatically balanced (which helped us to select amounts of reactants given to students), second, reaction was automatically formatted. Using Edit/Copy reaction to clipboard as RTF command and pasting it into quiz document we get all digits automatically and properly formatted:
6FeSO4 + K2Cr2O7 + 7H2SO4 → 3Fe2(SO4)3 + Cr2(SO4)3 + K2SO4 + 7H2O
That was even more than we asked for, so after pasting reaction into question we had to remove stoichiometric coefficients, so that reaction remained skeletal.
We have already selected that question will tell about 1.2 g of FeSO4, it was time to select amount of milliliters of dichromate solution. We put 1.2 g into appropriate EBAS edit field. Program automatically calculated stoichiometric amount of dichromate.
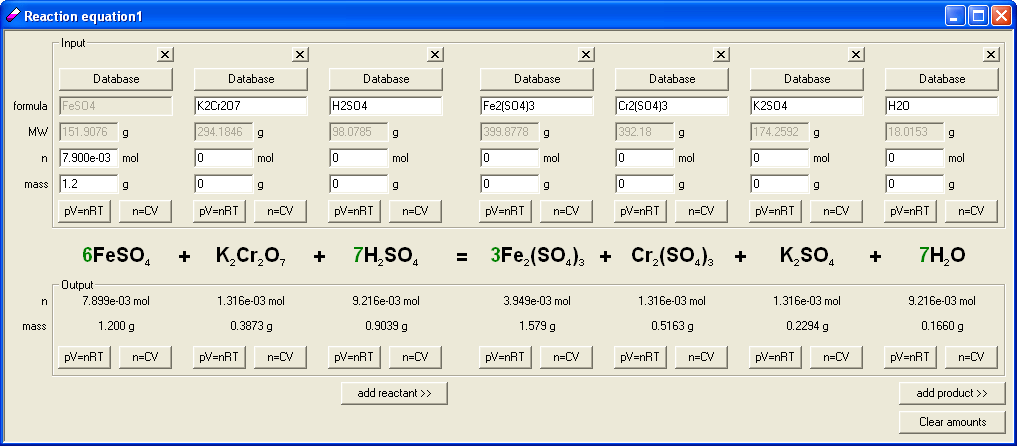
Built in concentration calculator allows to easily calculate volumes/concentrations of reagents, so we clicked n=CV button in the output frame and entered 1 as solution concentration, to find out how many milliliters of 1 M solution will stoichiometrically react with the sulfate. It turned out that we need 1.317 mL. This volume looks too small so we changed solution concentration to 0.1 M. That gave 13.17 mL which looks much better. Knowing stoichiometric amount of reagent we selected to use in different versions of the question volumes of 7, 9, 11, 13, 15 and 17 mL; this way we made sure it is the limiting reagent question as planned. As the reaction was already entered we prepared the answer key. To do so we clicked n=CV button in the input frame and entered 0.1 M as concentration, then 7 as a volume. Program automatically calculated number of moles of substance. Clicking on Use button we get back to the reaction were amount of iron (II) sulfate was displayed in red (which means it was in excess) and output frame contained already calculated mass of iron (III) sulfate - 0.8397 g.
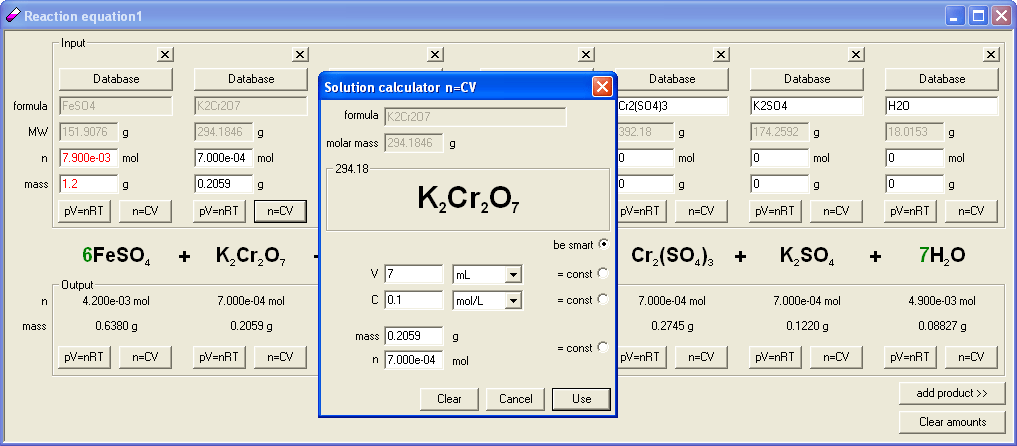
To calculate answers to questions with other volumes, it was enough to click several times on the n=CV button and to enter just new volumes as program remembers previously used concentration. Entering question, formatting skeletal reaction, choosing reasonable amount of reagents and preparing answer key took less then 3 minutes, including time for the discussion of the dichromate solution volume.
For the second question teacher decided to ask about volume of NO2 produced when copper is dissolved in nitric acid. To solve the question students had to balance redox reaction (already balanced during one of the lessons) and to show that they remember what gas evolves during dissolution (this is tricky, as usually what evolves is a mixture, but they have seen the experiment in which brown gas was produced). Ideal gas law was covered much earlier and was added here just to make sure they will not forget what they have learnt earlier. (As we were later told it started usual whining "you have not told us that will be on the quiz"). Ideal gas equation and values of R constant are hanging on the wall of the classroom, so students see them all the time, even during quizzes.
Question was worded as:
Piece of copper wire of ... g mass was dissolved in excess 15M nitric acid. What volume of gas was collected in the 25°C and 1009 hPa pressure. Answer: ............... L.
Six versions were prepared for printing, differing in the amount of copper (selected as 1.11, 1.41, 2.13, 2.52, 3.08 and 3.88 grams). To prepare answer key we have again entered skeletal reaction into EBAS (Cu + HNO3 = NO2 + Cu(NO3)2 + H2O) using New from string command again. Reaction was automatically balanced, and when the mass of copper was entered (we started with 1.11 g version) mass and number of moles of NO2 were automatically calculated. But we were interested in volume, so we clicked pV=nRT button below NO2 in the output frame, selected kPa as the pressure units (no hPa in the program), entered 100.9 (converting between hPa and kPa in memory) and 25 as temperature - and volume of the gas (0.8584 L) was calculated automatically.
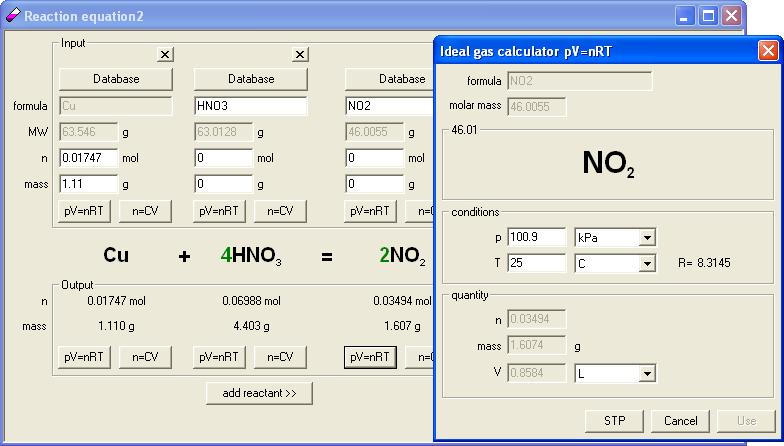
To check other answers it was enough to change mass of the copper and reopen ideal gas calculator - temperature and pressure entered are remembered by the program, so all answers were immediately ready. Everything was done so fast, that we decided to slightly modify original idea and to vary not only copper mass, but also temperature of the gas - entering 20, 22, 24, 26, 28 and 30°C, just because it was possible and easy to do. Once again, modification of printouts and answer key was very fast and took about 2 minutes.
Test was administered without any problems. We were not present during grading, but we were told that everything went smooth.



