You have 100 mL of 1 M ammonia solution (pKa=9.25). What volume of 1 M hydrochloric acid is needed to prepare buffer with pH=9.5?
Adding hydrochloric acid to the solution of ammonia (base) we create a conjugate acid NH4+. Ratio of their concentrations at pH 9.5 will be given by the Henderson-Hasselbalch equation.
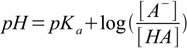 15.2
15.2If all added acid reacts with ammonia, amounts of conjugate acid NH4+ created and base NH3 left is defined by the stoichiometry of the neutralization reaction:
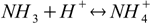 qbq2.1
qbq2.1and
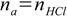 qbq2.2
qbq2.2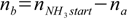 qbq2.3
qbq2.3qbq2.3 is equivalent to
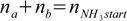 qbq2.4
qbq2.4where na and nb are number of moles of acid and conjugate base, nNH3start is number of moles of ammonia at the beginning (0.1 mole - n=CV) and nHCl is number of moles of acid added. We are using number of moles as it makes calculation much easier - this way we don't have to calculate how the concentrations change due to the dilution once the acid is added to original ammonia solution. We can do that thanks to the fact that in the Henderson-Hasselbalch equation volumes can be canceled out and replaced by mole ratio.
Putting pH=9.5 into the Henderson-Hasselbalch equation we get
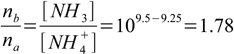 qbq2.5
qbq2.5Finally we have a set of equations qbq2.4 and qbq2.5. When solved they give na=0.036 and nb=0.064. From qbq2.2 we get that we have to add 0.036 mole of HCl, or 36 mL of 1 M solution.
We can use pH calculator to check the result. Create a solution containing both ammonia and hydrochloric acid. Enter 1 as both concentrations. From the drop down list above pH sign select Ca+Cb+Va+Vb. Enter 36 as acid volume and 100 as base volume. Perfect!
Note: if you need program that will help in buffer calculation, our pH calculator is not suited for the task, however, you can try our Buffer Maker - the buffer calculator. Buffer calculator was specifically designed to help in buffer preparation, so - given pH - it will automatically calculate recipe of any buffer you may need.



