Calculate pH of solution prepared from 0.1 mole of formic acid and 0.02 mole of NaOH diluted to 1L. pKa=3.75.
This is classic buffer question that we will solve using Henderson-Hasselbalch equation.
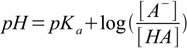 15.2
15.2We need to know concentration of HCOO- and HCOOH. Formic acid has pKa value larger than 2.5 (see pH of buffer section) so we can assume stoichiometry is solely responsible for concentrations of both acid and conjugate base. If so, after neutralization has taken place [HCOOH]=0.1-0.02=0.08 M and [HCOO-]=0.02 M. Putting these values into Henderson-Hasselbalch equation we get:
pH=3.75+log(0.02/0.08)=3.15.
Using pH calculator we get 3.17. Our rule of thumb (the one about pKa>=2.5) states that difference between real pH and calculated pH will be acceptable, not zero.



