Calculate pH of 0.1M potassium hydrogen oxalate. pKa1=1.25, pKa2=4.27.
This question - if done by hand - requires either approximate method (that gives questionable results) or tedious calculations.
In amphiprotic salt section we have derived equation 12.9
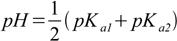 12.9
12.9According to the analysis we have done (results shown in the table) equation 12.9 gives good results for more concentrated solutions. 0.01 M is a little bit too diluted for the precise result.
pH=1/2(1.25+4.27)=2.76.
Let's check this result using pH calculator. To create potassium hydrogen oxalate solution you have to select oxalic acid and potassium hydroxide and to enter 0.1 as both concentrations.
pH calculator shows 2.86 - and this is the correct result. Approximate method is very fast - but gives only very rough results.



