A solution of 35.00% zinc nitrate has a density of 1.3678 g/mL. What is the volume of a sample of this solution that contains 25.00g of zinc nitrate?
Our starting point is the definition 6.1 of mass percentage:
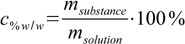 1.1
1.1Rearranging this equation we can easily calculate mass of the solution containing 25.00 g of zinc nitrate: msolution=25.00×100/35.00=71.43 g.
The problem is, we are asked not about mass, but about volume... However, knowing mass and density we can calculate volume rearranging density definition to:
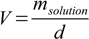 fvmpq1.1
fvmpq1.1And the volume V=71.43/1.3678=52.22 mL.
To find the answer using concentration conversion calculator select zinc nitrate from the database, enter mass of solid (25) and lock it clicking on the radio button right to the number of moles, then enter concentration % w/w (35). Both solution volume and mass wil be calculated and the correct density will be taken from the database.
In the unregistered (or light) version of CASC there is no zinc nitrate in database - you have to enter its formula manually, then enter mass of solid, lock it, enter known density and - finally - enter mass percentage. It takes less time than reading this description.



