What is pH of 0.0002M HI solution?
What will be pH if 1 mL of the above was added to 999 mL of pure water?
HI is a strong acid so we can assume it is fully dissociated.
3×10-4 M solution is concentrated enough to allow us to neglect water dissociation (see table in strong acid section). Thus we can use 7.6 equation
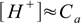 7.6
7.6and the pH calculated is -log(3×10-4)=3.70.
After the solution has been diluted 1000 times HI concentration is 2×10-7 M and we are in the area where water dissociation may spoil results of the 7.6 equation. Thus it wil be better to use the equation 7.4
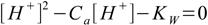 7.4
7.4and calculated pH=6.62 (you know how to solve quadratic equation, don't you?).
Using equation 7.6 we will get pH=6.70. Difference is not large - but clearly visible.
As additional confirmation our pH calculator shows exactly the same results - 3.70 and 6.62.



