Use acetic acid and sodium acetate to prepare 0.01 M buffer solution with pH 4.50. pKa=4.75.
We can easily calculate ratio of concentrations of acid and conjugate base, by plugging known values of pH and pKa into the Henderson-Hasselbalch equation and rearranging:
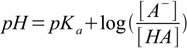 15.2
15.2[A-]/[HA] = 10pH-pKa = 104.50-4.75 = 0.562
0.01 M buffer means sum of concentrations of acetic acid and acetate is 0.01 M:
[HA] + [A-] = 0.01 M
We have two equations in two unknowns, solving them requires just a basic algebra skills and yields
[HA]=0.0064 M
[A-]=0.0036 M



