Calculate the amount of water needed to prepare 6.00 M solution from 25 mL of 12.00 M sulfuric acid.
Assuming this is a simple dilution we can try to solve question using equation 12.2:
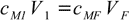 12.2
12.2cM1=12.00 M, V1=25 mL, cM2=6.00 M. Solving for VF we get VF=12.00×25/6.00=50 mL. As we already had 25 mL of solution, we have to add 50-25=25 mL of water.
Is it correct result?
Only approximately. Volumes are not additive, especially when there are huge changes in the concentration of the main solute. Adding 25 mL of water to 25 mL of 12 M sulfuric acid we will get slightly less than 49 mL of the final solution. This means that the error in our calculation is a little above 2%. To avoid this problem it is enough to fill up solution to 50 mL, but we were specifically asked about the volume of water needed. Correct result (calculated using dilution calculator which takes density changes into account automatically) is 26.08 mL.

