Calculate the amount of water needed to prepare 0.25 M solution from 25 mL of 6.00 M sulfuric acid.
This is a simple dilution question, that we can try to solve using equation 12.2:
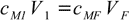 12.2
12.2We have cM1=6.00 M, V1=25 mL and cMF=0.25 M. Solving for VF we get VF=6.00×25/0.25=600 mL. This is the final volume, but we already had 25 mL of solution, so we have to add 600-25=575 mL of water.
Such an approach - although in most cases precise enough - can lead to errors, if the solutions are more concentrated. See the next dilution question.



