A 50 mL sample with a mass of 50.230 g was evaporated to dryness, the resulting residue mass was 0.453 g. Find the molar concentration of the NaCl solution.
We will use definition 6.1 of molar concentration:
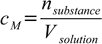 6.1
6.1We know the volume - 50 mL, we know mass of the solute - we don't know number of moles of solute, but it can be found from equation 10.3:
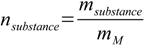 10.3
10.3Molar mass of NaCl is 58.44 g/mol, so c=0.453/58.44/0.05=0.155 M (0.05 stands for 0.05 L - volume was given in mL).
Mass of the sample given in the question is not necessary for calculations. This question was posted at the chemicalforums with sample mass given as 50.320 g, which was probably a typo.



