Molar concentration (molarity) definition
Molarity - or more correctly molar concentration, often denoted by M - is defined as
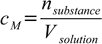 6.1
6.1and expressed in [mole/L] units. This is the most often used concentration unit, molar concentrations are often measured with highest precision and for most analytical applications this is the preferred way of expressing concentration as it makes stoichiometric calculations much easier.
The only drawback is that molar concentration depends on the temperature - when the solution gets warm it's volume in most cases increase and molar concentration goes down. 5 deg C temperature difference leads to concentration change in 0.1% range - so it can be a source of error of the same range as volume readings on the precise burette.
In old books and papers you may sometimes find concentration expressed as M/500 - which means 1 mol per 500 litres of solution (thus M/500 means 0.002 mol/L).
For example of molarity conversion see percentage to molarity section.



