I just purchased one of your programs. What do I need to do to use it?
First of all - download the program from our site, from the download page. Install it.
Registration key should have been attached to the order confirmation mail. Save it in the directory where the program is installed, most likely c:\Program files (x86)\ChemBuddy\program name. If the attachement was not named, save it using name regkey.txt. Alternatively open registration key with Windows notepad, copy content to the clipboard, start the ChemBuddy program, select File/Register from the menu, paste registration key content into edit field and click OK:
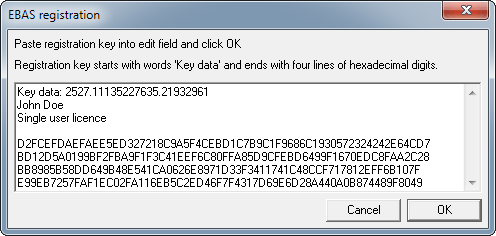
Please note copy/paste method may not work for registration keys containing characters that are outside of standard ASCII set.
In the case of ChemBuddy suite registration key will work for all programs. You may either register each program individually, or save the registration key in the c:\Program Files\ChemBuddy directory.
Program displays "Can't save the registration key" message and requires registering each time it is run. What to do?
This happens on some Windows configurations and is related to the way Windows installs programs and grants access rights to the directories used by the program.
First of all - check, if saving the registration key as regkey.txt file directly in the directory where the program is installed (typically a subdirectory of c:\Program files (x86)\ChemBuddy) doesn't help (note the file needs such access rights as to be readable by the user trying to run the program).
If not - try creating a directory ChemBuddy\ProgramName (be it BATE, CASC, EBAS or BufferMaker) in the directory c:\Users\(user name)\AppData\Local (also, one of our users reported using c:\Users\(user name)\AppData\Roaming worked on his Windows configuration) and then registering the program. Note you need the access rights to that directory.
How do I contact you?
Please use our feedback page.
It seems there is an error in BATE calculations - titration curve for strong base titrated with phosphoric acid has only two end-points, and they are in the wrong places!
That's what we thought at first too. However, everything is OK. Phosphoric acid is rather weak and to some extent behaves as if it had only two dissociations steps, hence only two end points. Strange localisation of these end points is an artefact of the way we have defined titration percentage. Note, that while we could use different definition, it will not help - just strange titration curves will be calculated in other situations. Compare discussion of titration of polyprotic acids at www.titrations.info.
Is it possible for BATE to calculate titration curve for NH4+ ion?
Yes. Just enter it as acid. However, ionic strength calculations are at the moment based on the assumption that undissociated molecules are not charged, so results of the calculated ionic strentgh can be misleading. For ignored or forced ionic strength calculations results will be correct.
What source did you use to obtain the pKb value of NaOH? As a result of this value, the pH of an equi-molar mixture of HCl and NaOH is not 7.00. This is a concern because our texts for general chemistry always teach that the equivalence point for a strong acid - strong base titration will be 7.00!
Good point. Most books don't give any values of Kb for strong bases. At the same time compilations of equilibrium constants (like Handbook of Chemical Equilibria in Analytical Chemistry, Kotrly and Sucha, Ellis Horwood Ltd. 1985) give stabilities of hydroxo-complexes of Na+ and K+ ions.
For complexation reaction
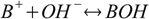
stability constant is defined as
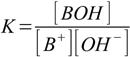
At the same time dissociation reaction is
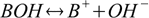
and the dissociation constant is defined as
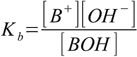
It is obvious that both reactions are undistinguishable and Kb = K-1. No matter whether we call this reaction dissociation, ion pairing, or complexation, it has the same effect on the final pH.
Logarithms of stability constants for KOH and NaOH are -0.5 and -0.2 respectively so pKb values are 0.5 and 0.2. Both bases - albeit strong - are weaker than it is commonly believed.
Where can I get more information about acid base equilibrium and pH and titration curve calculations?
First of all - try our pH lectures.
If our lectures and questions are not enough, try google. Google for "acid base equilibrium" or "pH calculation". There are many on-line courses that will show up.
If you are too lazy to Google by yourself try great collection of general chemistry resources at General Chemistry Online.
Where to find information about temperature for CASC density tables?
This information is not given in the program, however, you can compare density for pure water (0 concentration) with this table:
| density | temperature |
|---|---|
| 0.99913 g/mL | 15 deg C |
| 0.99880 g/mL | 17 deg C |
| 0.99862 g/mL | 18 deg C |
| 0.99823 g/mL | 20 deg C |
Does one license cover the installation of your software on my home computer in addition to my office computer?
If you are owner of a Single User License, yes. That's in the point 1.3 of our End User License Agreement. You can have the program installed on as many computers as you want, you just can't run it on more than one at the same time.



