Calculate mass of phosphine PH3 evolving when 12g of calcium phosphide Ca3P2 is added to 20g of water.
This is a classic stoichiometry problem - limiting reagent question. As in the case of most questions in chemistry, you have to start with balancing chemical equation.
Create new reaction, clicking on the New button on the tool bar. Click once on the add reactant button and once on the add product button to prepare room for all reagents.
In the upper, input frame, enter formulas of both reactants (Ca3P2, H2O - you may select water from the program database if you want) and products (PH3 and Ca(OH)2 - note that you don't need any tricks to correctly display the formulas, just enter letter, digits, and parentheses).
Program does its part of the work and displays balanced equation and calculated molar masses.
Now it's time for stoichiometry - enter known amounts of reagents - 12 into mass edit field below edit field with calcium phosphide formula and 20 into edit field below water formula.
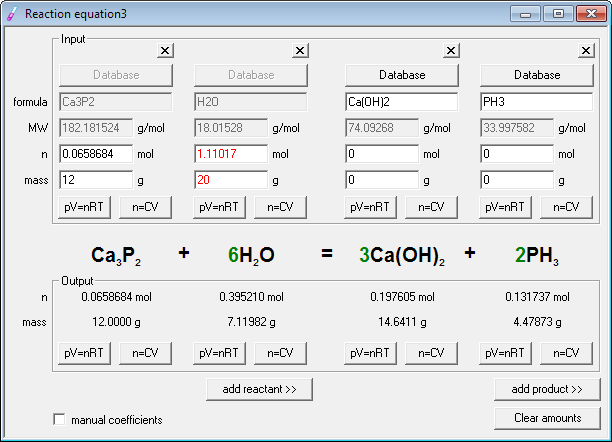
Ready! Entered amount of water is displayed in red, to signal it is in excess. Calcium phosphide is a limiting reagent. Stoichiometric amount of phosphine evolved is displayed in the lower, output frame below phosphine formula - and it reads 4.479 g. In fact we have only two significant digits in data, so it should read 4.5g, but program is somewhat generous when it comes to displaying digits.
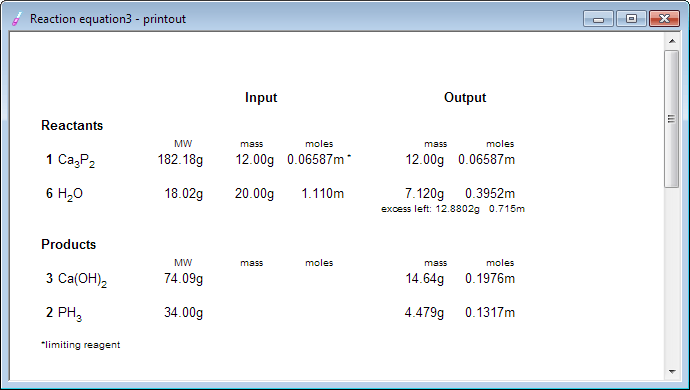
At this moment we can take a look at the reaction summary - or even print it, just in case:
However, phosphine is a gas - it will be nice to know what is its volume. You may use ideal gas calculator to check it.



