pH calculator program - Base Acid Titration and Equilibria - dissociation constants pKa and pKb
Here are some of the values of weak and strong acids and bases dissociation constants used by BATE when calculating pH of the solution and concetrations of all ions present.
For the definitions of Kan constants scroll down the page.
| name | formula | pKan |
|---|---|---|
| acetic (ethanoic) acid | CH3COOH | 4.75 |
| citric acid | C6H8O7 | 3.13 |
| 4.76 | ||
| 6.40 | ||
| carbonic acid | H2CO3 | 6.37 |
| 10.25 | ||
| hydrochloric acid* | HCl | -4 |
| hydrogen sulfide | H2S | 7.04 |
| 11.96 | ||
| nitric acid* | HNO3 | -1 |
| oxalic acid | (COOH)2 | 1.25 |
| 4.27 | ||
| perchloric acid* | HClO4 | -7 |
| phenol | C6H5OH | 9.89 |
| phosphoric acid | H3PO4 | 2.15 |
| 7.20 | ||
| 12.35 | ||
| sulphuric acid* | H2SO4 | -3 |
| 1.99 |
* see diclaimer below pKb table.
For the definitions of Kbn constants scroll down the page.
Note that ammonia and most organic bases release OH- ions due to hydrolysis, not dissociation. On the contrary inorganic bases - like NaOH, KOH, LiOH, Ca(OH)2 - increase pH dissociating.
| name | formula | pKbn |
|---|---|---|
| ammonia | NH3 | 4.75 |
| calcium hydroxide | Ca(OH)2 | 2.43 |
| 1.4 | ||
| lithium hydroxide | LiOH | -0.36 |
| methylamine | CH3NH2 | 3.36 |
| ethylamine | C2H5NH2N | 3.25 |
| potassium hydroxide | KOH | 0.5 |
| sodium hydroxide | NaOH | 0.2 |
| aniline | C6H5NH2 | 9.4 |
Disclaimer - accuracy of the values shown, especially for the strong acids, is questionable. Depending on the source pKa for HCl is given as -3, -4 or even -7. These values are usually not measured but calculated from thermodynamical data and should not be treated too seriously. Besides, difference between pKa=-1 and pKa=-10 starts to influence calculation results for the solutions with very high ionic strengths, such calculations are dubious in any case. As for pKb values of strong bases - NaOH, KOH, LiOH, Ca(OH)2 - pleas read the explanation in our FAQ section.
pKa and pKb values have been taken from various books and internet sources. Please remember that only some of them are included in the trial version database, but you can always enter them manually for calculations.
For the reactions of dissociation of acid:
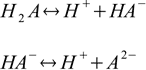
stepwise dissociation constants are defined as

For the reactions of dissociation of base:
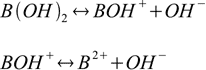
stepwise dissociation constants are defined as

Next dissociation steps are trated the same way. Dissociation can be also described by overall constants, as well as base dissociation constants or protonation constants. They are all defined in the help file accompanying BATE.



